Anti-Malaria Drug Backed By India 'Temporarily Pauses' As WHO Expresses Safety Concerns
May 26, 2020 11:07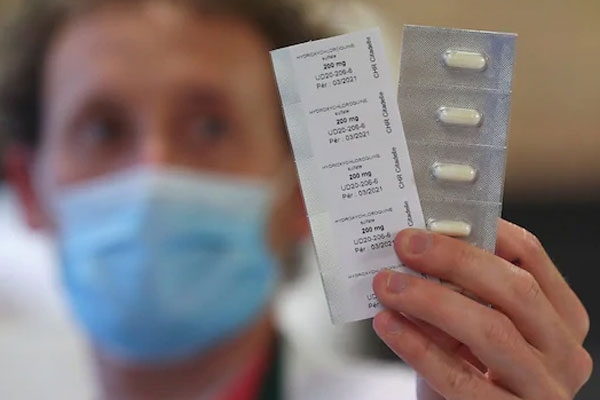
(Image source from: images.news18.com)
On Monday, the WHO, put a hold on anti-malarial drug hydroxychloroquine that was intended to being used for treating COVID-19.
After studying further, they found that Hydroxychloroquine and chloroquine are used for treating lupus, rheumatoid arthritis, for preventing and treating malaria. However, no strong tests proved if these were safe or effective for preventing or treating COVID-19.
Earlier, the Union Health Ministry had advised to use this medicine as a preventive from getting infected with Coronavirus.
Now, the WHO officials have decided to suspend the use of this medicine. They came up with this decision after reviewing the Lancet study that was published stating that this drug is linked to a possibility of death and heart ailments.
According to WHO director-general Tedros Adhanom Ghebreyesus, using hydroxychloroquine and chloroquine in Covid-19 is the biggest concern.
Further, he says, these drugs have been approved for treating malaria or autoimmune diseases. Besides, drug remdesivir and an HIV combination therapy are still being tested.
On Saturday, the WHO officials met and decided to review the available data on hydroxychloroquine and for now its use in the trial would be suspended.
WHO’s emergencies chief, Dr. Michael Ryan, said that there wasn’t any indication of safety problems with hydroxychloroquine while it was trialed by WHO. Now, this would be analyzed by the Statisticians.
The WHO is focusing on being safe after viewing the recent results of the studies so that they can be safe and move forward. In another two weeks, they are expecting to get results.
A couple of Departments and health officials gave a warning about the side effects of this drug. Last week, there was an announcement made by Trump about taking hydroxychloroquine although he was not tested positive for Covid-19.
The European Medicines Agency, the US Food and Drug Administration and his own administration had said that it was dangerous to use this drug for treating Covid-19 outside of hospital or research settings because of its side effects.
By Neha Makhija