(Image source from: economictimes.indiatimes.com)
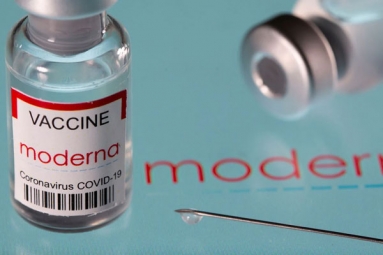
The Indian government has been keen to make several foreign vaccinations available in the market to meet the demand as there are crores of people left to get vaccinated. Indian pharma giant Cipla received permission to import the coronavirus vaccine Moderna for emergency use in the country. Cipla filed an application to waive all the bridging trials for the foreign vaccines. Moderna is expected to be 90 percent effective against coronavirus. Moderna is an mRNA vaccine like Pfizer. Albert Bourla, the CEO of Pfizer recently announced that Pfizer will be available in the Indian market very soon.
The Drugs Controller General of India (DCGI) granted permission for emergency usage of Moderna as per the provisions of the New Drugs and Clinical Trial Rules, 2019 under the Drugs and Cosmetics Act, 1940. Cipla will have to submit the safety assessment of the vaccine for seven days in the first beneficiaries who will take the vaccination shot. Central Drugs Standard Control Organisation (CDSCO) is favorable to grant permission to Cipla to import the Moderna vaccine. On June 27th, Moderna informed the Drugs Controller General of India that the government of USA agreed to donate a number of doses through COVAX to India and they are waiting for the approval from the Central Drugs Standard Control Organisation. After Covishield, Covaxin and Sputnik, Moderna is the fourth vaccine to be available in the Indian market.
By Siva Kumar